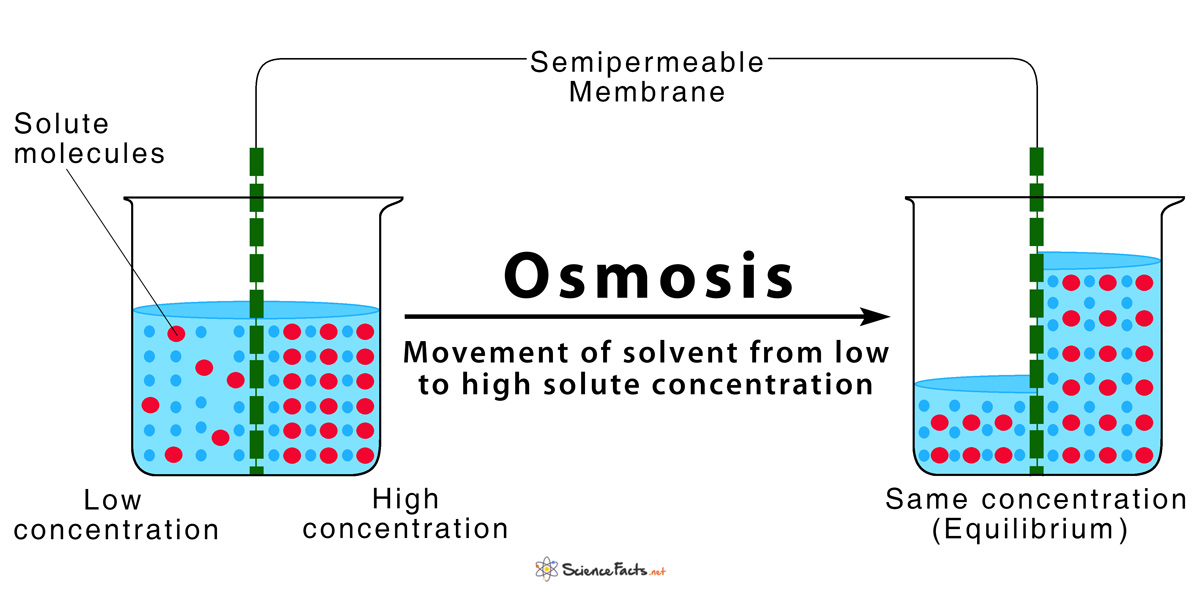
Osmotic Pressure Is Best Described As . osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. External pressure can be exerted on a solution to counter the flow of solvent; this tendency is called osmotic pressure. osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable.
from www.sciencefacts.net
External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. this tendency is called osmotic pressure.
Osmosis Definition and How Does it Occur (with Diagram)
Osmotic Pressure Is Best Described As osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. this tendency is called osmotic pressure. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Osmotic Pressure Is Best Described As osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. External pressure can be exerted on a solution to counter the flow of solvent; this tendency is called osmotic. Osmotic Pressure Is Best Described As.
From chem.libretexts.org
13.7 Osmotic Pressure Chemistry LibreTexts Osmotic Pressure Is Best Described As this tendency is called osmotic pressure. osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. External pressure can be exerted on a solution to counter the flow of. Osmotic Pressure Is Best Described As.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Osmotic Pressure Is Best Described As osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. this tendency is called osmotic pressure. osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane.. Osmotic Pressure Is Best Described As.
From infinitylearn.com
What is Osmosis Definition, Types, Osmosis Pressure andSignificance Osmotic Pressure Is Best Described As osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. External pressure can be exerted. Osmotic Pressure Is Best Described As.
From ar.inspiredpencil.com
Osmotic Pressure Equation Units Osmotic Pressure Is Best Described As External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. this tendency is called osmotic pressure. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores. Osmotic Pressure Is Best Described As.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Is Best Described As this tendency is called osmotic pressure. osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure, the amount of force applied to a solution. Osmotic Pressure Is Best Described As.
From slideplayer.com
Membrane Dynamics ppt download Osmotic Pressure Is Best Described As osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. this tendency is called osmotic pressure. osmotic pressure can be thought of as the pressure that would be. Osmotic Pressure Is Best Described As.
From exollfhbm.blob.core.windows.net
Osmotic Pressure Is Measured In at Robert Dang blog Osmotic Pressure Is Best Described As osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due. Osmotic Pressure Is Best Described As.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) Osmotic Pressure Is Best Described As osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or. Osmotic Pressure Is Best Described As.
From dauglas.afphila.com
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs Osmotic Pressure Is Best Described As this tendency is called osmotic pressure. osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. osmotic pressure is the pressure caused by a difference in the amounts. Osmotic Pressure Is Best Described As.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Is Best Described As External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. osmotic pressure, the amount of force applied to a solution that. Osmotic Pressure Is Best Described As.
From preparatorychemistry.com
Osmosis Osmotic Pressure Is Best Described As osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a. Osmotic Pressure Is Best Described As.
From www.slideserve.com
PPT Osmosis, Osmotic pressure and Molecular processes PowerPoint Osmotic Pressure Is Best Described As osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. this tendency is called osmotic pressure. osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure is the pressure a solvent exerts to prevent the inward. Osmotic Pressure Is Best Described As.
From www.chemistryland.com
Colligative Properties Osmotic Pressure Is Best Described As osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a semipermeable. this tendency is called osmotic pressure.. Osmotic Pressure Is Best Described As.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmotic Pressure Is Best Described As osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids). osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure, the amount of force applied to a solution that prevents solvent from moving across a. Osmotic Pressure Is Best Described As.
From www.sciencefacts.net
Reverse Osmosis Definition, Principle, and Applications Osmotic Pressure Is Best Described As External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference. Osmotic Pressure Is Best Described As.
From www.shutterstock.com
Osmotic Pressure Infographic Diagram Showing Fresh Stock Vector Osmotic Pressure Is Best Described As osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. External pressure can be exerted on a solution to counter the flow of solvent; osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. osmotic pressure, the amount of. Osmotic Pressure Is Best Described As.
From www.coastalwiki.org
Osmosis Coastal Wiki Osmotic Pressure Is Best Described As osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing. External pressure can be exerted on a. Osmotic Pressure Is Best Described As.